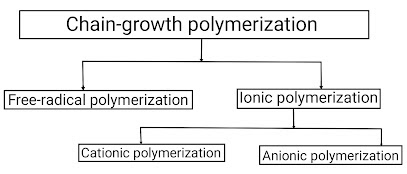
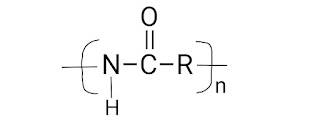
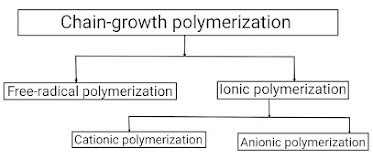
Chain Polymerization Mechanism

First of all, we learn about the basis of Polymerization techniques; " Polymerization technique is defined as the fundamental process by which low molecular weight compounds (Monomers) are converted into high molecular weight compounds (means, polymers) ". There are several polymerisation techniques are available but most of the polymerization techniques fall under two categories: Addition polymerization and Condensation polymerization The mechanism of chain polymerization reaction or the chemical reaction (or other process) is the reaction in which the products themselves promote or spread the reaction. In this, reactive product or by-product causes additional reactions to take place. (A series of events so related to each other that each one initiates the next) This chain polymerization mechanism occurs in three basic steps; 1) Chain Initiation 2) Chain Propagation 3) Chain Termination 1) Chain initiation – It is the first step in the chain-growth polyme...