Chain Growth Polymerization Or Addition Polymerization
Polymerization is a process in which thousands of smaller molecules called "monomers" join together to form larger molecules called "macromolecules/polymers". They have high molecular weight.
Several different categories of polymerization reactions exist, but the most notable of which are: step-growth polymerization and chain growth polymerization.
Chain-growth Polymerization
Chain-growth polymerization reaction is one of two processes to convert a monomer into a polymer.
"Chain-growth polymerization reaction is a polymerization technique where unsaturated monomer (means containing double and triple bonds in their molecular formula) molecules add onto the active site ( It can be radicals, positive ions and negative ions etc.) on a growing polymer chain one at a time".
Chain-growth polymerization reaction also known as Addition polymerization reaction.
Monomers used in chain growth polymerization reaction;
Ethylene/ethene [CH₂=CH₂]
Propylene [CH₃–CH=CH₂]
Butylene [CH₃–CH₂–CH₂=CH₂]
Ethyne [CH≡CH]
Propyne [CH₃–C≡CH]
Styrene [C₆H₅–CH=CH₂], etc.
Example of chain-growth polymers are Polyethylene (High density Polyethylene and Low density Polyethylene), Poly-vinyl chloride (PVC), Polystyrene, Polypropylene, Styrofoam (expanded polystyrene), etc.
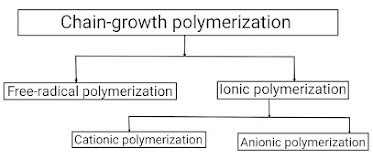
Addition polymerization occurs in four ways
• Free Radical Polymerization (Here, an active species arises as a "free radical".)
• Cationic Chain Polymerization (Here, an active species arises as positive ions)
• Anionic Chain Polymerization (Here, an active species arises as negative ions)
• Co-ordination Polymerization (Here, an active species arises as a co-ordination compounds)
Mechanism of Chain-growth Polymerization
☛ Usually an initiator compound reacts with the monomer to start the reaction, and the mechanism of chain growth polymerization consists of five phases, called "chain initiation", "chain propagation", "chain transfer", "chain inhibition" and "chain termination".
☛ Typically, chain growth polymerization reaction must contain chain initiation and chain propagation. Chain transfer, chain inhibition and chain termination do not always happen in a chain-growth polymerization.
(1)Chain initiation–
It is the first step in the chain-growth polymerization process that is required to initiate polymer chain growth. In this, first the initiator is converted into intermediate with the help of light, heat or catalyst. These intermediates can be atoms, ions, molecules or fragments of molecules. These initiators may be monofunctional or multifunctional or may be unsaturated in nature.
Once a reactive intermediate is generated, it can react with other stable molecules to form new reactive intermediates. The propagation of step involves the formation of new intermediate molecules or atoms or ions, etc.
(3)Chain termination–
In this step, all the intermediates generated from the first two steps disappear and no intermediate remains. Hence, termination lowers the concentration of intermediates from the system.
Hope you have found this article helpful!!
Do you have suggestions? Please write in comment box!!!
Feel free to comment if you have any queries!!






Comments
Post a Comment