Kinetics of Free Radical Polymerization
"Chain-growth polymerization or addition polymerization reaction is a polymerization technique where unsaturated monomer (means containing double and triple bonds in their molecular formula) molecules add onto the active site on a growing polymer chain one at a time".
Since, we know that depending on this active site present on the growing polymer chain, there are three types of addition polymerization:
free radical polymerization, ionic polymerization, and coordination polymerization.
Free radical polymerization is one of those types of additional polymerization in which the active site is free radical. In this, initiators are used to generate free radicals. Free radical is denoted as "M•".
➛ Initiator molecules are of the following types;
(a) Thermal initiators (such as peroxides, azo-compounds)
(b) Photochemical initiators (such as Disulfides, ketones, Azo, Peroxides,etc.)
(c) Redox initiators (such as Fenton reagent)
(d) Ionising initiators ( such as X-ray, r-rays, hจ, etc.)
Since free radical polymerization is a type of addition polymerization reaction, therefore it occurs in three steps as addition polymerization; Chain initiation, Chain propagation and Chain termination.
☛ Let us look at these three steps one by one;
Chain Initiation
In this, first the initiator is converted into free intermediate (or active species) with the help of light, heat or catalyst. Here, the intermediate is the free radical. Once free radicals are formed, they react with the monomer molecule. In polymer chemistry, the first free radical reacts with a monomer molecule to form a new free radical species in the initiation step.
Chain Propagation
From the initiation step, the radical reacts with other stable monomer molecules to extend the polymer chain and form new free radicals. Thus, the propagation step involves the formation of new free radicals.
Chain Termination
This is the final step of the chain reaction cycle. In this step, all the intermediates or free radicals generated from the first two steps disappear and no free radical remains. Termination can happen through a combination (or coupling) or disproportionation. Here, we will look at the termination by coupling of two growing polymer chains;
The final rate expression for the decomposition of monomer molecules in case of free radical polymerization reaction is given below,
For Example
Polyethylene or poly-ethene is a polymer whose monomer is ethene or ethylene. Polyethylene is produced from ethylene monomer by free radical polymerization. The chemical structure of ethene and Poly-ethene is given below,
Polymerization of ethene into polythene is done in 3 steps;
(a)Initiation
Benzoyl peroxide is used as an initiator molecule.
(b)Propagation
Propagating chain polymer,
R–CH₂–(CH₂–CH₂)ₙ–CH₂•
(c)Termination
Termination by disproportionation;
R–CH₂–(CH₂–CH₂)ₙ–CH₂• + • R
–––––––––––––––>
R–CH₂–(CH₂–CH₂)ₙ–CH₂–R
Termination by coupling;
R–CH₂–(CH₂–CH₂)ₙ–CH₂• +
•CH₂–(CH₂–CH₂)ₙ–CH₂–R
–––––––––––––––––>
R–CH₂–(CH₂–CH₂)ₙ–CH₂–R
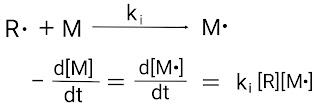













Comments
Post a Comment