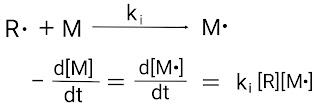Homopolymerization and Co-polymerization

" A polymers is defined as a large molecule (or macromolecule), formed by joining together thousands of smaller molecule units by chemical bonds ". These smaller molecules that interact with each other to form a larger molecule are called " Monomers ". And in polymer chemistry, a reaction by which smaller molecules (called monomers) react with each other to form a larger molecule (called polymers), is called " Polymerization ". Polymers are the high molecular mass compounds. We know that a polymer can be formed from only one type of monomer unit or from two or more than two different types of monomer units. Based on that, there are two types of polymerization techniques; namely Homopolymerization reaction and Copolymerization reaction. (In my previous posts, we have seen two types of polymerization techniques, namely chain-growth polymerization reaction and step-growth polymerization reaction classified on the basis of saturated or unsaturated monom...