Kinetic chain length for different addition polymerization reaction
First of all we should know, What is Kinetic Chain Length? And why do we need it?
After that, we will know what the kinetic chain length will be for the various addition polymerization processes. Let us first see what is the length of the kinetic chain.
Kinetic chain length–
The kinetic chain length of a polymer, in polymer chemistry, is the average number of units called monomers added to a growing polymer chain during chain-growth polymerization.
OR
The kinetic chain length of a polymer is defined as the average number of monomer units that react with active centers such as radicals, ions, etc, from the initiation phase to the termination phase.
Mathematical definition,
The kinetic chain length of a polymer is equal to the rate of the overall polymerization reaction divided by the rate of the initiation step in which the chain carriers are formed. Thus,
➛ "Average number of monomer units polymerized per chain initiated".
Kinetic chain length for addition polymerization reaction–
For the chain-growth polymerization or addition polymerization process, the kinetic chain length is defined as the ratio of the rate of the propagation step to the rate of initiation step.
And this is because both the monomer disappearance rate and the propagation step rate are the same for addition polymerization. In addition polymerization, the propagation step gives the overall rate of the polymerization process.
Upon steady state polymerization, since chains are initiated at the same rate as they are being terminated (Ri = Rt). Therefore the rate of initiation phase is equal to the rate of termination phase. Thus, the above relation becomes;
Kinetic chain length for free radical polymerization
For free radical polymerization, the rate of disappearance of monomer or the rate of propagation step is given as,
➩ Ri = kd.[I]
Thus, the kinetic chain length of free radical polymerization is,
Kinetic chain length for cationic polymerization
For cationic polymerization, the rate of disappearance of monomer or the rate of propagation step is given as,
Whereas the rate of initiation step is given as,
➩ Ri = ki[M][I⁺]
Thus, the kinetic chain length of cationic polymerization is,
Kinetic chain length for Anionic polymerization
For anionic polymerization, the rate of disappearance of monomer or the rate of propagation step is given as,
Whereas the rate of initiation step is given as,
➩ Ri = ki[M][I⁻]
Thus, the kinetic chain length of free radical polymerization is,
Hope you have found this article helpful!!
Do you have suggestions? Please write in comment box!!!
Feel free to comment if you have any queries!!








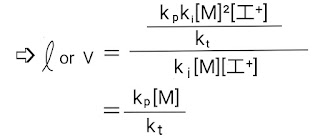






Comments
Post a Comment