Introduction To Polymer
Polymers forms a very important class of materials without which life seems very difficult. They are all around us in everyday use.
Polymers are very large molecules which are very essential for our existence. It is a main component of our food (starch, protein, etc.), our clothes (polyester, nylon, etc.), our home (wood cellulose, alkyd paint, etc.), and our body (poly (nucleic acid), protein, etc.).
Polymers are found in abundance on the Earth's surface; either in nature or artificially prepared. For example;
We use plastic bags, cards, paper, wood, clothing, paint and endless items that contain the basic chemical composition of polymers. Human and animal bodies are mainly made up of proteins and these proteins are the building blocks of life. They are also polymers.
Definition
The word polymer is a combination of two Greek words- "Poly+Mers"
Poly means 'many'
Or Mers means 'parts of unit'
☆ Poly + Mers ➝ many parts of unit
➛ Thus, "A polymers is defined as a large molecule (or macromolecule), formed by joining together thousands of smaller molecule units by chemical bonds (or covalent bonds)".
These smaller molecules that interact with each other to form a larger molecule are called "Monomers".
In polymer chemistry, a reaction by which smaller molecules (called monomers) react with each other to form a larger molecule (called polymers), is called "Polymerization".
For example- Polyethylene is an example of polymer in which the monomer molecule is ethene and a process by which these small molecules of ethene are linked together to form a polymer molecule polyethylene, is called Polymerization process.
Classification of Polymer
Polymers can have different chemical composition, different physical properties, different mechanical behavior and different thermal properties, etc., and based on these properties polymers can be classified in different ways. Such as;
• Origin or Source- Natural, Synthetic and semi-synthetic polymer
• Structure- Linear, Branch, cross-link pr 3D network polymer
• Number of monomer- Homopolymer, hetero-polymer or Copolymer
• Backbone chain- Organic and inorganic polymer
• Polymerization techniques- Addition polymers and condensation polymers
• Functionality- Mono-functional, Bi-functional, Tri-functional polymers
• Tacticity- Atactic, Isotactic and Syndiotactic polymers
• Physical properties or molecular forces- Elastomers (or rubbers), Fibres, Plastics (Thermoplastic and Thermosetting polymers)
• Polarity- Polar polymers and non-polar polymers
• Crystallinity- Amorphous polymer, Crystalline and semi-crystalline polymers
Molecular weight of Polymers
"The molecular weight of a polymer is defined as the sum of the atomic weights of each atom in the molecules, which is present in the polymer". Since, polymer molecules are made of smaller molecules. Therefore, the length (and consequently the molecular weight) of the polymer chain depends on the number of monomers added to the polymer.
The polymer molecules are not always of the same length. These are of different chain lengths. So we calculate the average molecular weight of the polymer instead of the molecular weight. It is calculated on two basis;
(a) Number average molecular weight
If N₁,N₂,N₃,........ are the numbers of macromolecules (or polymers) of different chain-length with molecular masses M₁,M₂,M₃,......... respectively then the number average molecular weight of polymer is given by a formula;
(b) Weight average molecular weight ;
If m₁,m₂,m₃,........ are the masses of macromolecules (or polymers) with molecular masses M₁,M₂,M₃,......... respectively then the weight average molecular weight is given by a formula;
➛ The mass average molecular weight is always greater than the number average molecular weight. It is sometimes equal to but not less than the number average molecular weight.
➛ Mₙ= ∑x¡M¡ & Mw= ∑w¡M¡
Here,
x¡ denotes mole fraction
w¡ denotes weight fraction
Poly-dispersity index
To understand how the molecular weight is distributed within polymers, we study about the polydispersity index. The polydispersity index measures the distribution of molecular mass in a given polymer sample.
"The polydispersity index is defined as the ratio of weight average molecular weight to the number average molecular weight of a polymer". It is denoted by a symbol " Đ /PDI ".
Case-1;
When, PDI or Đ=1 then the polymer is mono-disperse. In this case, both the number average molecular weight and the weight average molecular weight are the same.
➩ { Mₙ= Mw }
Nearly all the natural polymers are mono-disperse or uniform.
Case-2;
When, PDI or Đ ≻1 then the polymer is poly-disperse. In this case, weight average molecular weight is higher than the number average molecular weight. Polymers are of various chain-lengths.
➩ { Mₙ ≻ Mw }
Nearly all the synthetic or man-made polymer are poly-disperse.
Degree of Polymerization
Another term that is used in polymer chemistry is the degree of polymerization. "The degree of polymerization (or DP) is defined as the total number of monomeric units present in a macromolecule or polymer". It is calculated by a simple formula,
But in reality, polymer molecules have chains of different lengths so the average value of the degree of polymerization is used.
Generally, the degree of polymerization is a key characteristic of polymers that determines the physical properties of that polymeric material.
Since the molecular weight of a polymer is calculated on two bases, namely the number average and the mass average molecular weight, therefore the degree of polymerization is also calculated on this basis.
☆ "The number average degree of polymerization is the ratio of number average molecular weight to the molecular weight of monomer"
☆ "The weight average degree of polymerization is defined as the ratio of weight average molecular weight to the molecular weight of monomer".
Example of polymers and their monomers
(1) Poly-ethene, also called polyethylene, is composed of ethene or ethylene monomer. Commonly called "Plastic".
 |
| Polythene |
(2) Similarly, Poly-propene, also called poly-propylene, is composed of ethene or ethylene monomer.
(3) Polystyrene, aromatic polymers, is composed of styrene monomer.
(4) PVC (or Poly-vinyl chloride), is composed of vinyl chloride monomer.
(5) Teflon, or PTFE➛ Poly-tetrafluoroethylene, is composed of TFE (or tetra-fluoro-ethylene) monomer.
(6) Butyl Rubber, sometimes called as "butyl" only, is composed of Isobutylene monomer.
(7) PMMA (or Polymethyl-methacrylate) whose monomer unit is methyl-methacrylate. It is more transparent than glass.
(8) Nylon-6, belongs to polyamide family, is composed of Caprolactam monomer.
(9) Nylon-66 ,also belongs to Polyamide family, is composed of two monomer units namely adipic acid and hexa-methylene-diamene.
Hope you have found this article helpful!!
Do you have suggestions? Please write in comment box!!!
Feel free to comment if you have any queries!!



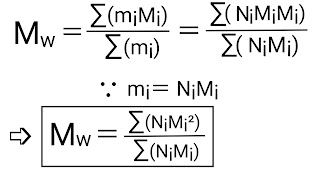






Comments
Post a Comment