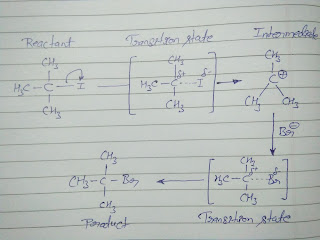
Comparison between Transition state Vs Intermediate
Comparison between transition state and intermediate
- Transition states are highly unstable while intermediate molecules are stable in nature.
- Transition states are impossible to separate from the reaction mixture while intermediate molecules can be separated from the reaction mixture easily.
- Transition states are having higher energy than compared to intermediate molecules.
- Transition states have short lifetime (measured in femto-seconds) while the reaction intermediates have a finite lifetime (a few nanosecond or many days).
- Transition states are formed in elementary reactions while intermediates are not formed in elementary reactions
- Intermediates are formed in one elementary step of the reaction and then consumed in another elementary step of the reaction. While transition state is present in each step of a chemical reaction.
Let's take a picture to clearly understand about transition state and intermediate and their formation;
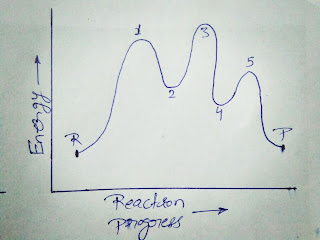
Let's have a look on a picture given below which is a question,
Que- Calculate the number of transition states, intermediates and elementary steps involved in the reaction whose Reaction Energy diagram is given?
Solution -
Here,
P - stands for product
R - stands for reactant
As we know that the points at which the energy of molecules is maximum in reaction energy diagram, are known as transition states while the points at which energy of molecules is minimum in reaction energy diagram, are known as intermediates.
Points having maximum energy -1,3,5
Points having minimum energy -2,4
Thus, the reaction will have two intermediate molecules and three transition states, as well as the reaction being completed in three elementary steps.
☛ We can say that the total number of elementary steps involved in the reaction is equal to the total number of transition states present in the reaction.
Hope you have found this article helpful!!
Do you have suggestions? Please write in comment box!!!
Feel free to comment if you have any queries!!





Comments
Post a Comment